James L. Drinkwater – Head of GMP compliance
- GMP facility & aseptic process design
- Contamination Control Strategy (CCS) preparation support
- Consultation on GMP requirements and compliance
Current Good manufacturing practice (cGMP) requires an understanding of process and associated science to manage contamination risks in sterile medicinal products and Advanced Therapeutic Medicinal (ATMPs) product manufacturing.

Dr. Birte Scharf – Senior Scientist GMP Compliance
- GMP compliant process integration
- Aseptic Containment lead (ACS)
- R&D projects: science and compliance
At FZ, we specialize in developing customized processes that meet regulatory requirements and expectations to ensure GMP-compliant processes for the aseptic manufacture of sterile drug products and ATMPs.

Dr. Hussein Bachir – Scientist GMP Compliance
- GMP compliant process integration
- Scientific conferences
- GMP training; science and process
Protecting medicinal products and therapies from contamination starts with design and technologies based on good science and process understanding. At Franz Ziel we aim for excellence in these principles to meet GMP compliance.

Dr. Marina Gole – Senior Scientist GMP Compliance
- Microbiological and chemistry laboratory services lead
- GMP compliant process integration
- Author for the customer magazine
Our primary goal is the safety and well-being of patients. For this purpose, we design excellent machines and technologies that meet the requirements and regulations of Good Manufacturing Practice (GMP).
We are providing GMP Compliance support across teams for project success.
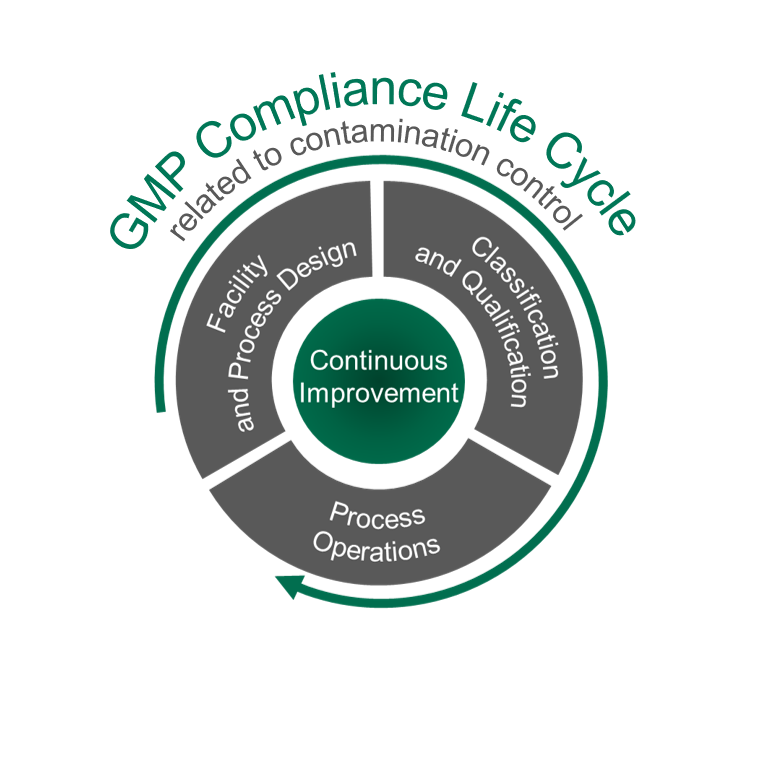
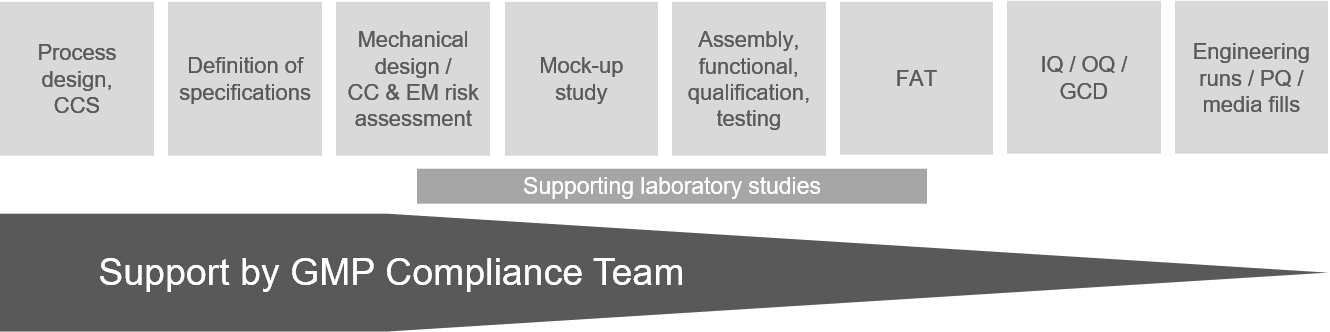

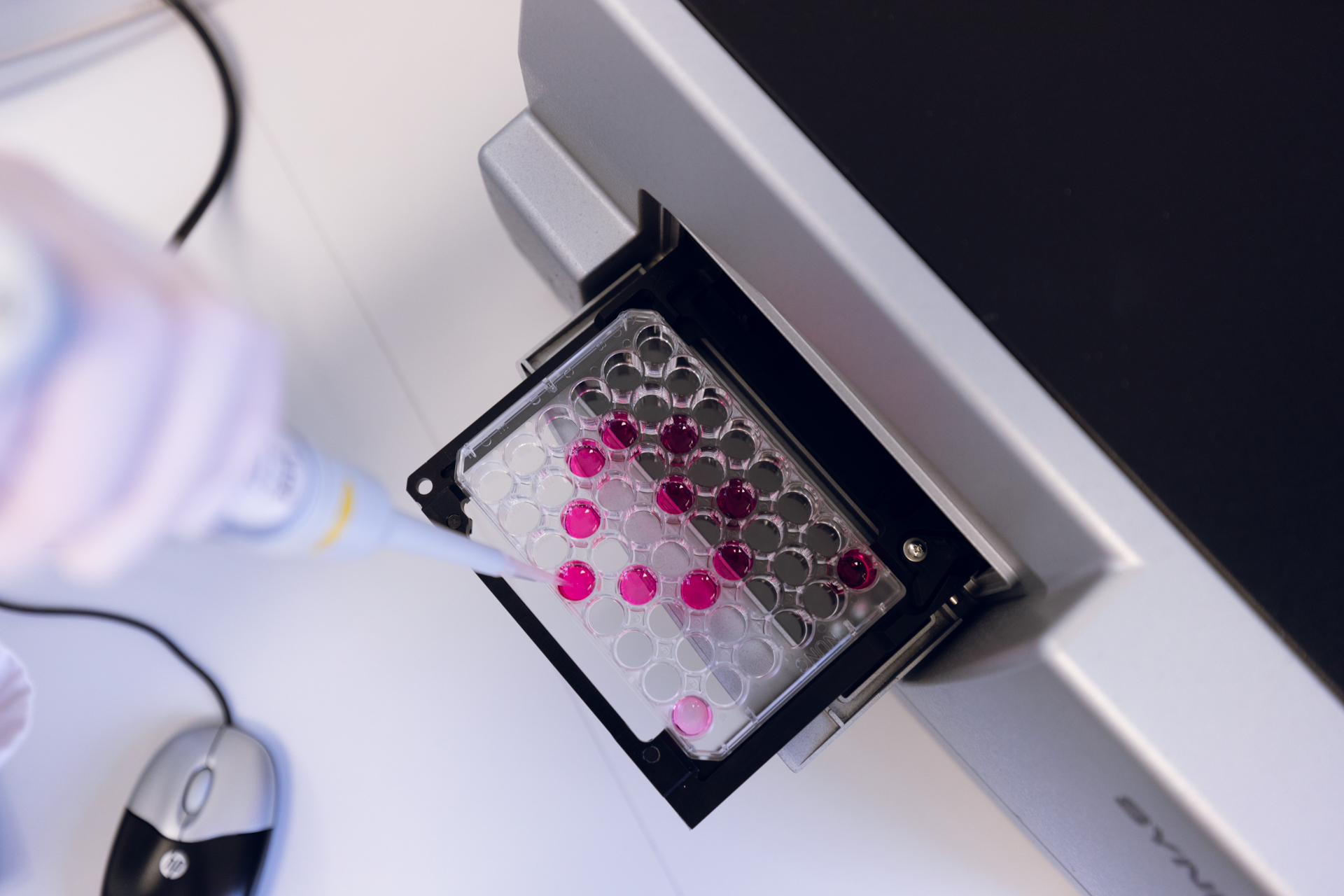
With an in-house microbiological and chemical laboratory, FZ is supporting decision and qualification steps in customer projects.
You will find more information about our aseptic processing technologies department here: