Isolator / Rabs
Barrier systems for different requirements for personal protection, product protection or a combination of personal and product protection as standard or special designs.
Barrier systems for different requirements for personal protection, product protection or a combination of personal and product protection as standard or special designs.
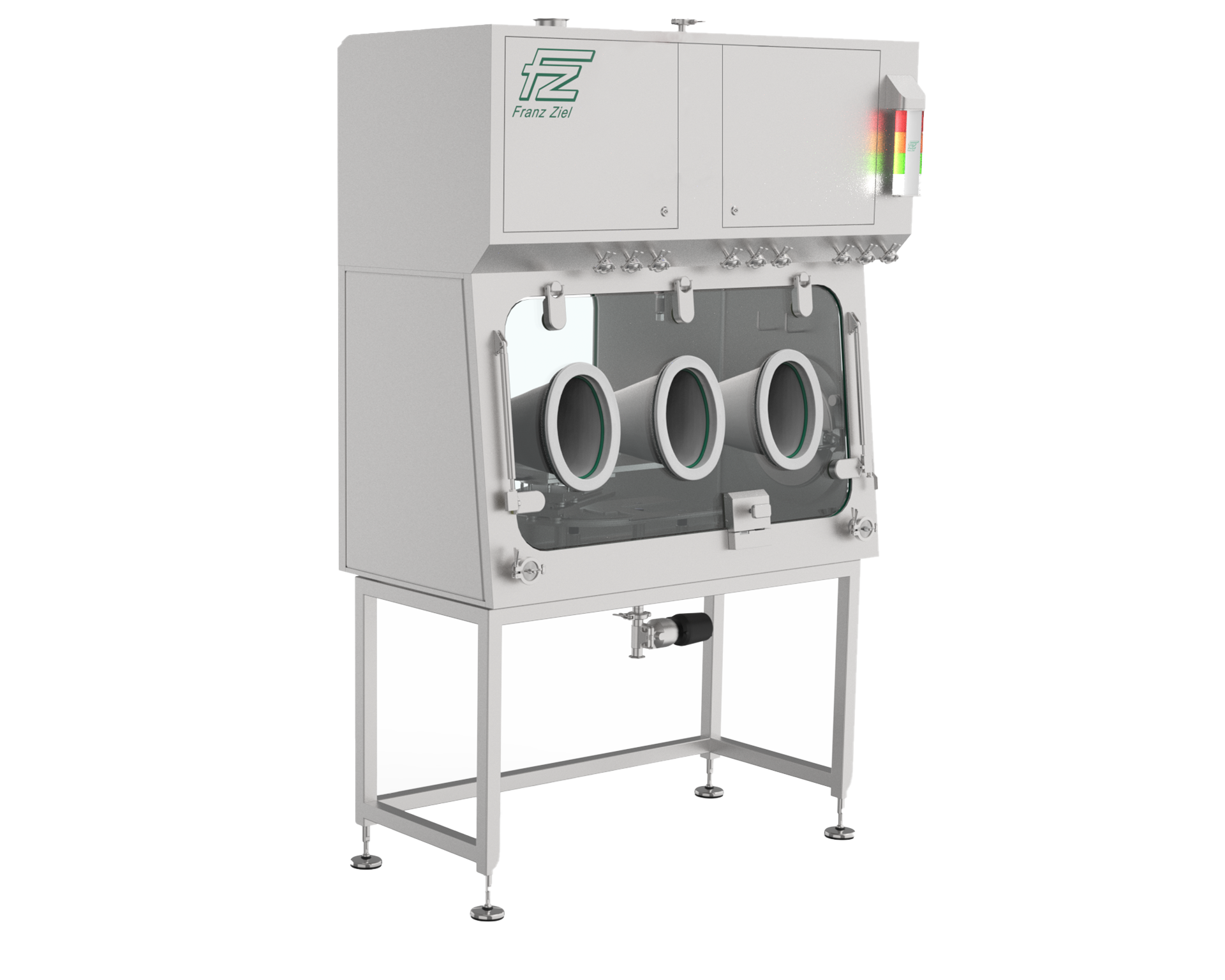
PPI isolators provide personal protection when handling toxic substances.
Personal protection is achieved through a barrier (isolator housing with gloves) between the operator and the toxic substance.
In addition, a defined negative pressure is generated in the working area of the isolator by a HEPA supply and exhaust air filter system, thus increasing safety for the operator. The filter elements are changed without contamination.


Isolators in compact design with product protection as a housing for aseptic processes, e.g. Filling, loading and unloading of freeze dryers. With a total height of 3,000 mm, no additional technical area is required.
Product protection is achieved through a barrier (isolator housing with gloves) between the operator and the product.

Isolators with personal and product protection as a housing for aseptic / toxic processes, e.g. Filling, loading and unloading of freeze dryers. Personal and product protection is achieved through a barrier (isolator housing with gloves) between the operator and the product. Product safety is achieved through the Laminar Air Flow System including a controlled overpressure control.

The Flex Lab Isolator (FLI) is a flexibly configurable isolator for clinical studies, small batches or other aseptic laboratory applications. The isolator is suitable for aseptic, bio-hazard and high-potent applications. Key features are a product protection (Grade A) and an operator protection when handling bio-hazard or toxic substances (option). Fast material transfers can be maintained with the integrated Rapid Decon Hatch (RDH). The FLI is independent from the on-site HVAC system.

Sterility testing isolators with product protection for the sterility test under aseptic and optionally aseptic / toxic conditions. A Rapid Decon Hatch (RDH) can be connected as an option. Product protection is achieved through a barrier (isolator housing with gloves) between the operator and the product. Product safety is achieved through the Laminar Air Flow System including a controlled overpressure control.

cRABS as a housing for aseptic processes, e.g. Filling, loading and unloading of freeze dryers. Product protection is achieved through a barrier (cRABS housing with gloves) between the operator and the product. The product safety is created by the Laminar Air Flow System including a controlled overpressure control.
oRABS as a housing for aseptic processes, e.g. Filling, loading and unloading of freeze dryers. Product protection is achieved by a barrier (oRABS housing with gloves) between the operator and the product. Product protection is created by the Laminar Air Flow System (type: active oRABS).

Cellefill is a turnkey, small batch, vial filling machine with an integrated containment solution.
The GMP compliant system has been designed by aseptic fill/finish experts Flexicon Liquid Filling and containment experts Franz Ziel.
As a fully integrated solution for the biopharmaceutical industry, Cellefill maximises production capabilities through recipe driven operation and delivers enhanced levels of process assurance to accelerate your time to market.
Cellefill optimises your batch security with highly accurate peristaltic pump performance and integrated environmental monitoring.
For further information follow this link: https://www.wmfts.com/de-de/marken/flexicon-keimfreies-abfuellen-von-fluessigkeiten/vollautomatische-systeme/vial-filling-machines/

Top standards in safety and flexibility – discover flexfill, a state-of-the-art filling and closing solution for ready-to-use syringes, vials, and cartridges. Designed to meet the demanding needs of small to large pharmaceutical manufacturers and Contract Development and Manufacturing Organizations (CDMOs), flexfill delivers unmatched flexibility and reliability.
The flexfill system offers a standardized, qualified, and validated machine concept that supports your pharmaceutical production goals. Its modular design enables 80% pre-defined configuration, allowing for fast deployment and commissioning. This streamlined setup minimizes lead time for qualification and validation, helping you meet regulatory requirements quickly and reliably. The long and intensive collaboration between groninger and
Franz Ziel ensures an optimal project flow and perfectly coordinated process, as if from a single source.
With flexfill you can choose from pre-configured modules to customize your system according to specific needs. Benefit from:
The pre-designed and configurable filling line with isolator offers you unrivalled delivery times for cutting-edge technology for pharmaceutical fill and finish solutions for ready-to-use (RTU) syringes, vials and cartridges. The standardization and modularization of industry-leading groninger and Franz Ziel technology enables shortened times to production start-up while meeting the highest standards of safety and flexibility. flexfill can be equipped with the different containment solutions of Franz Ziel GmbH to meet the highest quality as well as GMP requirements and regulations.
Further information about flexfill: https://www.groninger-group.com/en/pharma/flexfill/