Accessories
Innovative technologies to support the aseptic process.
Innovative technologies to support the aseptic process.

The Glove Testing System enables for Annex 1 compliant wireless and tubeless leak testing of gloves installed in Isolators or RABS.
The GTS with the latest software technology offers an extremely flexible and fully integrable solution for glove testing as part of glove management.
Characteristics:
Key features:
Gloves constitute the most susceptible link in the containment barrier systems. Gloves are the most critical part in aseptic manufacturing environment because they are in direct contact with the operator and for aseptic manipulations hence gloves are the main source of potential contamination.
A faulty Glove can breach the integrity of isolator and contaminate the product.
According to Draft Annex 1 ‘’Integrity testing of the barrier systems, and leak testing of the glove system and the isolator should be performed using a methodology demonstrated to be suitable for the task and criticality. The testing should be performed at defined periods, at a minimum at the beginning and end of each batch, and should include a visual inspection following any intervention that may affect the integrity of the system’’
Routine and frequent leak testing of glove/sleeve system is highly recommended. FZ provides one stop solution for protection of your gloves and products.
More information needed? Just get in touch with us
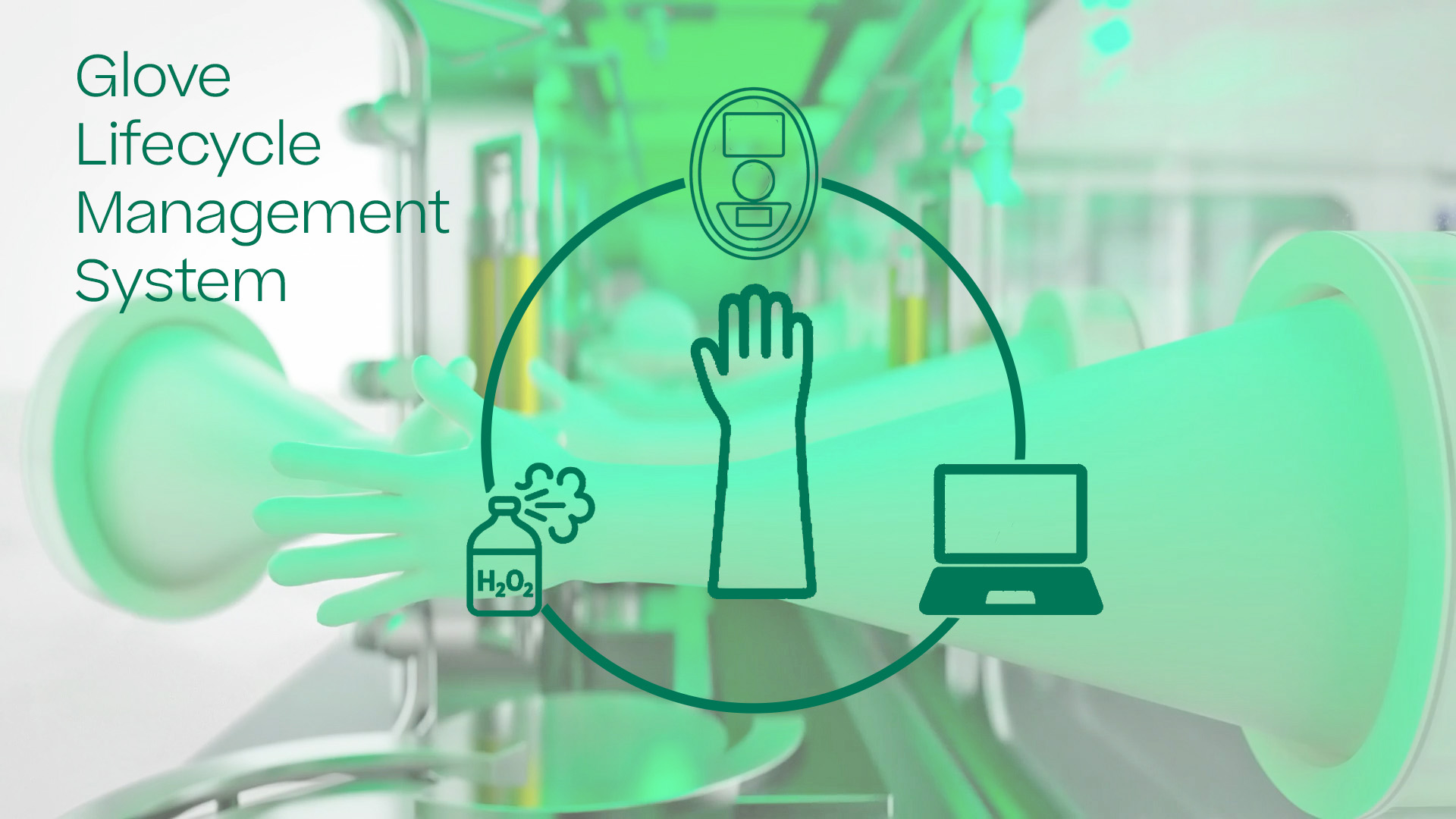
Your gloves – all in your hand
Can you manage one of the vulnerable parts in aseptic processing? When working with Isolators or RABS, Gloves are currently in use nearly everywhere, but they involve some risks.
You can reduce risks by using an efficient tracking and management solution. With the Franz Ziel Glove Lifecycle Management System, you can track every step your gloves take within your production facility. You can track everything for each glove individually, from sterilization over installation in the isolator to integrity testing with the GTS (glove testing system), as well as monitoring how many biodecontamination cycles the glove has undergone. Full transparency and full control for your equipment.
Interested in more? Contact our sales (sales@ziel-gmbh.com) for more information about Glove Lifecycle Management.