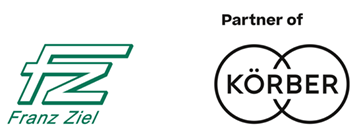
Sophisticated functions with modern technology – the Rapid Decon Station (RDS) made by Franz Ziel provides you with a flexible solution for material transfer into aseptic areas of the pharmaceutical production process. Designed for the pharmaceutical industry, the RDS fulfils various GMP-compliant functions to support the production process with the aseptic transfer of expendable materials and usage components/articles.
More information or individual demonstration? Shure, just get in contact with us:
One for all
When several lines or systems need to be supplied with material, our RDS is the ideal solution. While production processes are running, bio-decontamination processes can simultaneously proceed in the RDS and material can be prepared to be fed into the production process. The material can then be yielded via RTP (Rapid Transfer Ports) straight to where it is required.

Decontamination of non-autoclavable components in less than 30 minutes
Franz Ziel’s RDS enables you to decontaminate non-autoclavable components such as tools or materials in the shortest possible time. These components are then available for insertion into the system via rapid transfer ports in less than 30 minutes.
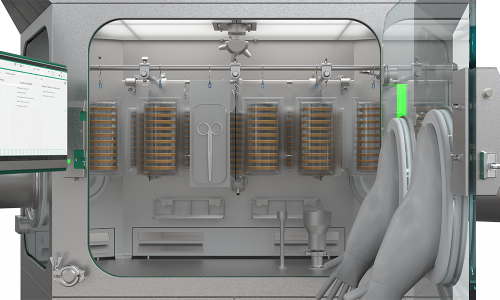
Flexibility and adaptability: always fitting
The RDS has easily interchangeable side panes in which different Rapid Transfer Port (RTP) sizes can be integrated. Should systems have different RTPs (size or manufacturer), they can all be operated with the same RDS.

Discover the benefits of the RDS – just download the RDS brochure (PDF):
Modern and ergonomic design
The RDS has been specially adapted to the pharmaceutical industry’s requirements. Years of experience and research have also shaped the design: clear lines, smooth surfaces, hygienic configuration and a lateral door all render cleaning easier. The design allows optimum access from three sides without blocking additional space on the sides. The interior with its large volume and large viewing windows makes optimum use of the small footprint.

Simultaneous decontamination of beta container
The RDS enables simultaneous decontamination of connected beta container (transfer container). This allows cleaning processes to be simplified and accelerated. Production processes and the introduction of material into systems can be planned precisely and carried out easily.
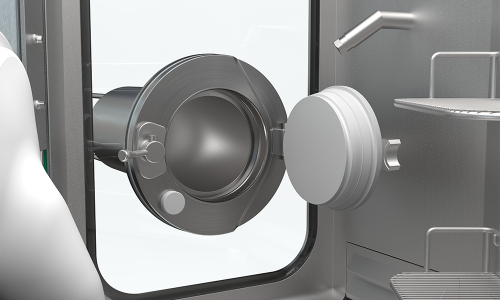
No on-site heating, ventilation or air conditioning (HVAC) required
The RDS operates self-sufficient, independent of the on-site heating, ventilation and air-conditioning technology (HVAC). As all required technology is already integrated, our RDS is neutral to the air (temperature) in the room.
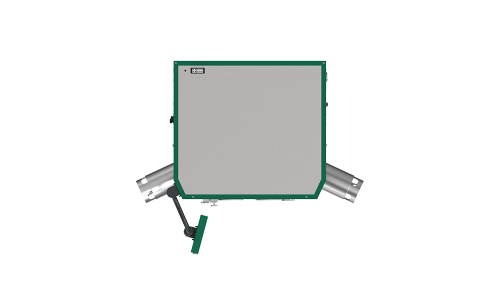
„Plug & Play”: RDS only requires a power connection and compressed air
You only need electricity and compressed air to operate the RDS. These can simply be provided via the existing plugs or plug connections. Assembly/installation on site is therefore quick and easy.
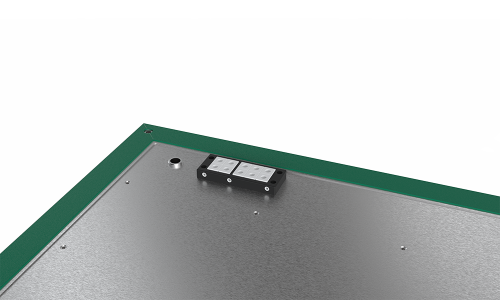
Material transfer wherever you need it
The RDS can be installed and operated anywhere in a cleanroom class D or C. This gives you more flexibility regarding the choice of location as well as the simultaneous operation of several systems.
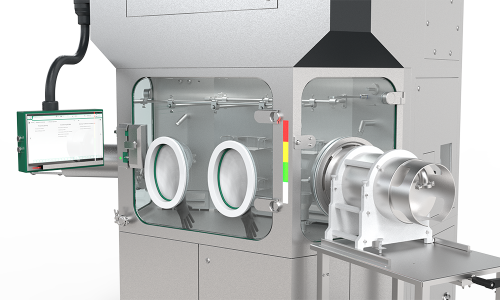
Usability as Grade A material store
Flexibility and immediate availability for tools and consumables according the requirements of Annex 1. You can also use the RDS as a secure Grade A material storage to be able to react quickly during the pharma production process.

Convenient, proven, reliable
The RDS combines modern technology and proven functions to offer users maximum convenience. For the safety of products and operators and to ensure that all processes can be carried out in confidence, reliable technology and functions are highly important. The RDS is therefore GMP Annex-compliant and ready for use, with all relevant functions and components already documented in a URS (user requirement specification).
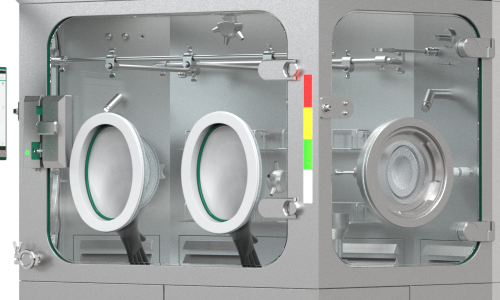
Compatible with Franz Ziel‘s GTS Glove Testing System
Our Glove Testing System (GTS) provides you with a simple manner to test gloves installed in isolators, RABS or RDS for leaks (GMP-compliant according to Annex 1) wirelessly and without hoses. Thanks to the user-friendly software, you can configure and save several facilities in the system and test them successively. The disk size can be variably adapted to the RTPs of various manufacturers. You thus benefit from standardized reports and complete documentation in an “all systems in one”- software package.

Rapid Decon Station: for large and small pharmaceutical companies alike
Would you like further information or a demo of the RDS?
Reach out to us: sales@ziel-gmbh.com or +49 2543 2335-0.
Or just fill out the contact form and we will contact you to make an appointment: